Archive: 2026/01
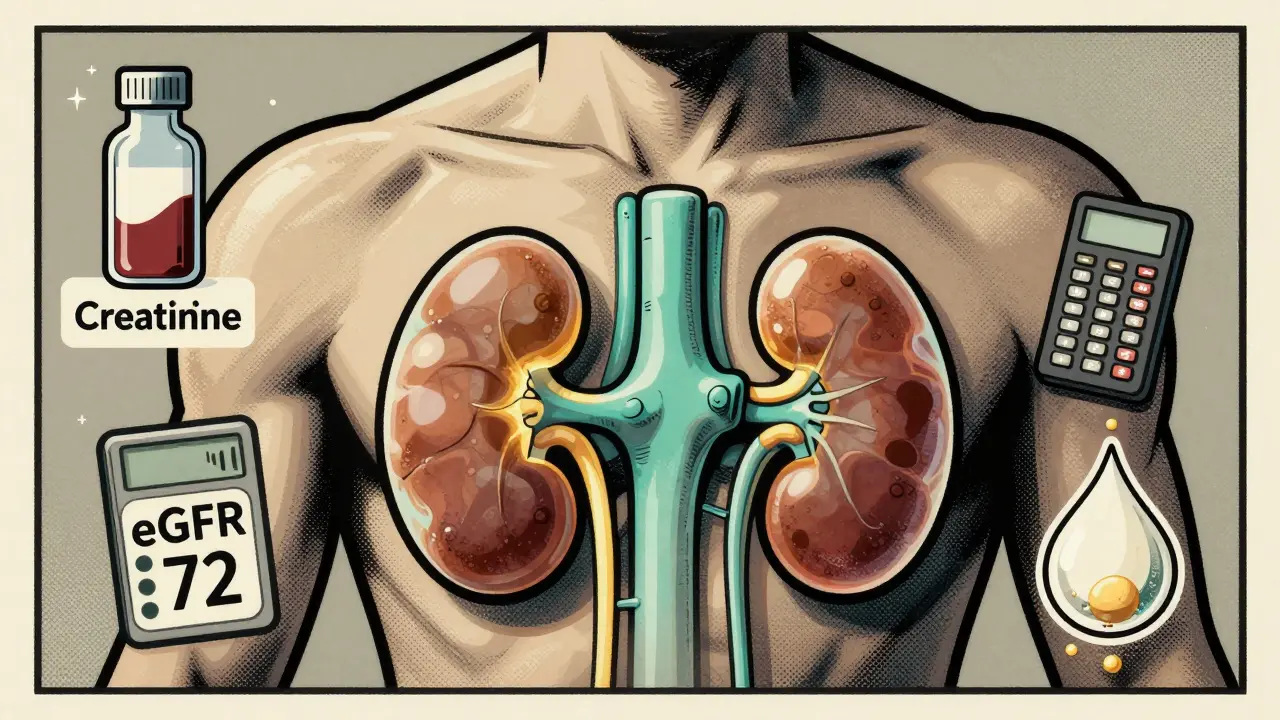
Learn how creatinine, GFR, and urinalysis tests detect early kidney damage - even before symptoms appear. Essential info for anyone with diabetes, high blood pressure, or over 40.
Learn how pharmacy systems accurately identify generic and brand-name drugs using NDC codes, therapeutic equivalence ratings, and FDA guidelines. Best practices for safety, compliance, and patient trust.
Atopic dermatitis flares are triggered by dry air, sweat, and irritants. Emollient therapy repairs the skin barrier, reduces water loss, and prevents flares when used correctly-twice daily, right after bathing. It’s the foundation of all eczema treatment.
Learn how family and caregivers can effectively support medication adherence with practical tools, routines, and communication strategies backed by clinical research and real-world experience.
Learn how to safely dispose of used needles and sharps from injected medications. Avoid risks of infection and injury by following UK-approved disposal methods, container guidelines, and free drop-off options.
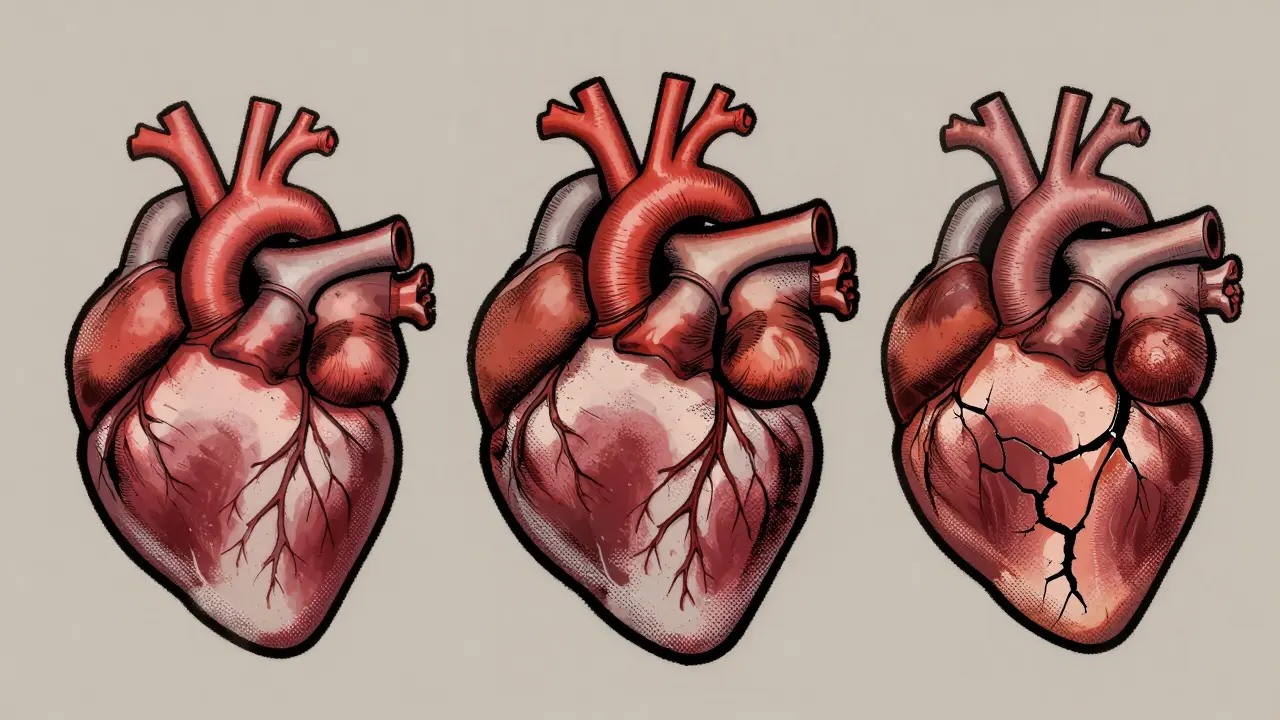
Learn the key differences between dilated, hypertrophic, and restrictive cardiomyopathy-how they affect the heart, what causes them, and how they’re treated. Understand symptoms, survival rates, and the latest advances in diagnosis and therapy.
Compounded medications offer custom drug formulas for patients who can't use standard prescriptions due to allergies, dosage needs, or swallowing issues. Learn when they're necessary, how to find a safe pharmacy, and the risks involved.
Medication errors happen in both hospitals and retail pharmacies, but the types, frequency, and risks differ greatly. Learn how errors occur, why they’re dangerous, and what you can do to protect yourself.
Patient-reported outcomes let you share your real-life experience with medications, helping regulators and drugmakers spot side effects doctors might miss. Your daily feedback improves safety for everyone.
The FDA doesn't rate biosimilars like generics-they go through a rigorous science-based approval process to prove they're highly similar to the original biologic. Here's how it works, what interchangeable means, and why U.S. standards are stricter than Europe's.
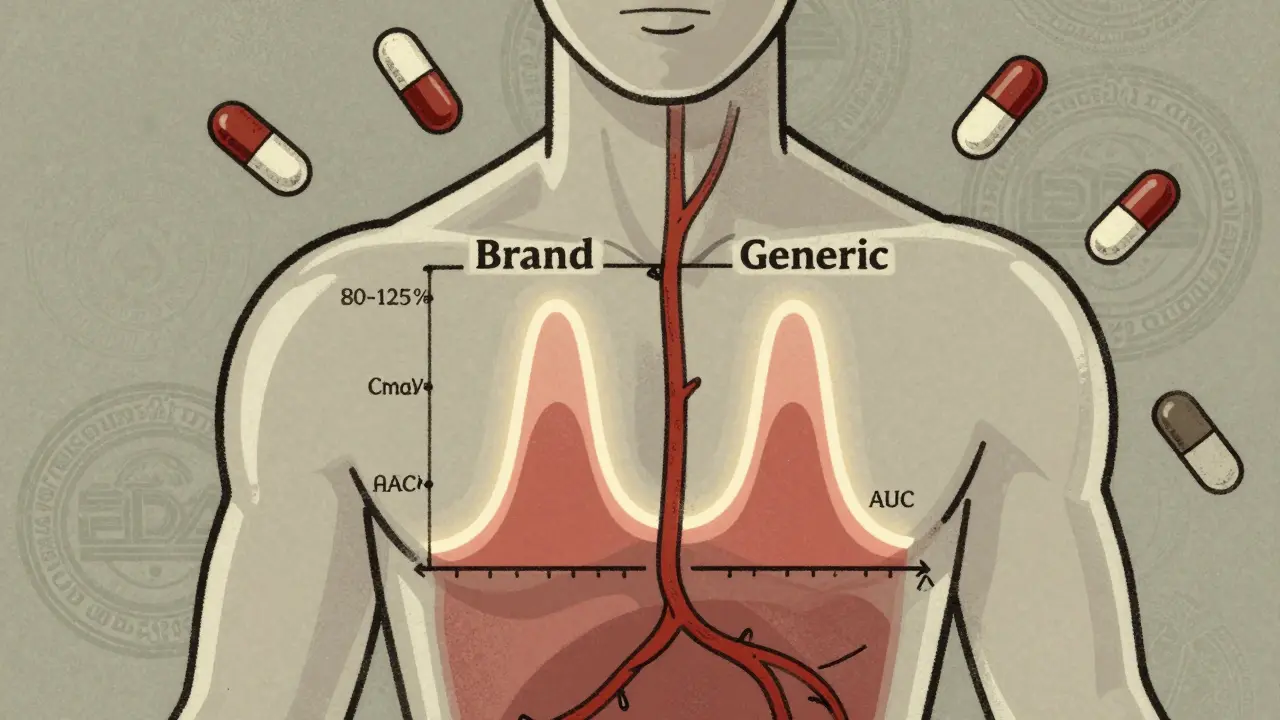
Learn how the FDA ensures generic drugs work just like brand-name versions through strict bioequivalence testing. Understand the science behind Cmax, AUC, and the 80-125% rule that guarantees safety and effectiveness.
Measuring patient education effectiveness isn't about what was taught-it's about what was understood. Learn how to track real, lasting understanding using simple, evidence-based methods that improve outcomes.