Tag: bioequivalence
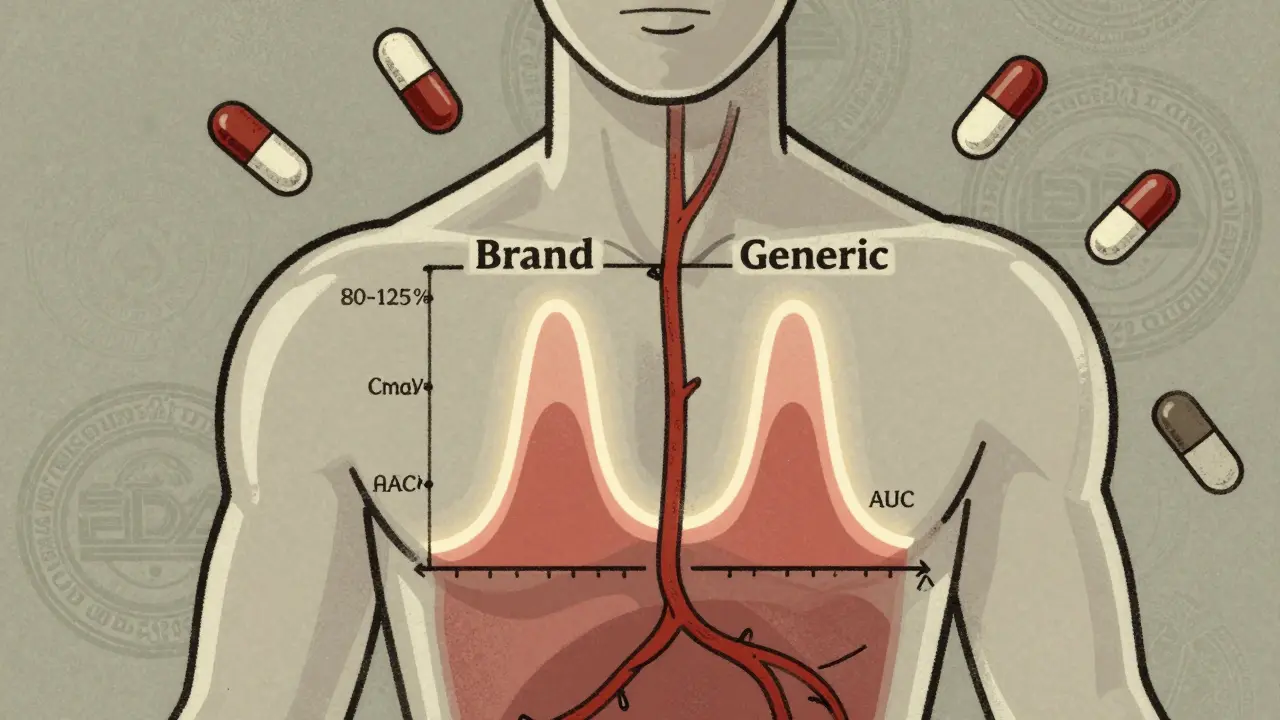
Learn how the FDA ensures generic drugs work just like brand-name versions through strict bioequivalence testing. Understand the science behind Cmax, AUC, and the 80-125% rule that guarantees safety and effectiveness.
An ANDA is the FDA's pathway for approving generic drugs without repeating costly clinical trials. It saves patients billions annually by ensuring safe, effective copies of brand-name medications enter the market quickly.
Cmax and AUC are the two key metrics used to prove generic drugs work the same as brand-name versions. Cmax measures peak concentration, while AUC measures total exposure. Both must fall within 80%-125% to ensure safety and effectiveness.