When you take a pill, injection, or inhaler, you expect it to work exactly as it should-every time. But drugs don’t stay perfect forever. Heat, humidity, and time can break them down. That’s why stability testing isn’t optional-it’s the backbone of drug safety. If a medication degrades before you use it, it might not work. Worse, it could harm you. That’s why regulators demand strict controls over temperature and time during testing. This isn’t just paperwork. It’s what keeps millions of patients safe.
What Stability Testing Actually Does
Stability testing answers one simple question: How long can this drug stay safe and effective under real-world conditions? It’s not about how well it works in a lab on day one. It’s about what happens after six months in a hot warehouse, a humid pharmacy shelf, or a patient’s medicine cabinet. The goal is to set a shelf life-like an expiration date-and define how the product must be stored.
This process is governed by global standards, mostly from the ICH Q1A(R2) guidelines. These rules were created in the early 2000s by regulators from the U.S., Europe, and Japan to make sure everyone tests drugs the same way. If a company skips this step or gets it wrong, the FDA or EMA can issue warning letters, block approval, or even recall the product. In 2022 alone, the FDA issued 27 warning letters tied to stability testing failures.
Temperature and Time: The Core Rules
There are three main types of stability tests, each with fixed temperature and time requirements. These aren’t suggestions-they’re legal requirements.
- Long-term testing: This is the real-world simulation. For most solid oral drugs, the standard is either 25°C ± 2°C with 60% RH ± 5% RH, or 30°C ± 2°C with 65% RH ± 5% RH. You run this test for at least 12 months before submitting a drug for approval. The FDA requires 12 months of data. The EMA lets you submit with 6 months if you’re using Option B, but you still need to finish the full 12 months later.
- Accelerated testing: This is the stress test. All drugs are exposed to 40°C ± 2°C and 75% RH ± 5% RH for six months. This isn’t meant to be realistic-it’s meant to speed up degradation. If the drug breaks down badly here, you know you’ve got a problem. This test predicts how the drug will behave over years in normal conditions. Studies show that 6 months at 40°C/75% RH roughly equals 24 months at 25°C/60% RH-for about 85% of small-molecule drugs.
- Intermediate testing: This one’s only needed if the accelerated test shows problems and you’re using the 25°C long-term condition. You run it at 30°C ± 2°C and 65% RH for six months. It’s a middle ground to help explain why the drug failed acceleration.
Refrigerated products follow different rules. Long-term storage is at 5°C ± 3°C for 12 months. Accelerated testing for these is done at 25°C/60% RH-not the 40°C used for room-temperature drugs. That’s because freezing and thawing can ruin biologics and injectables. The WHO makes this clear in Annex 10.
Global Climates, Different Rules
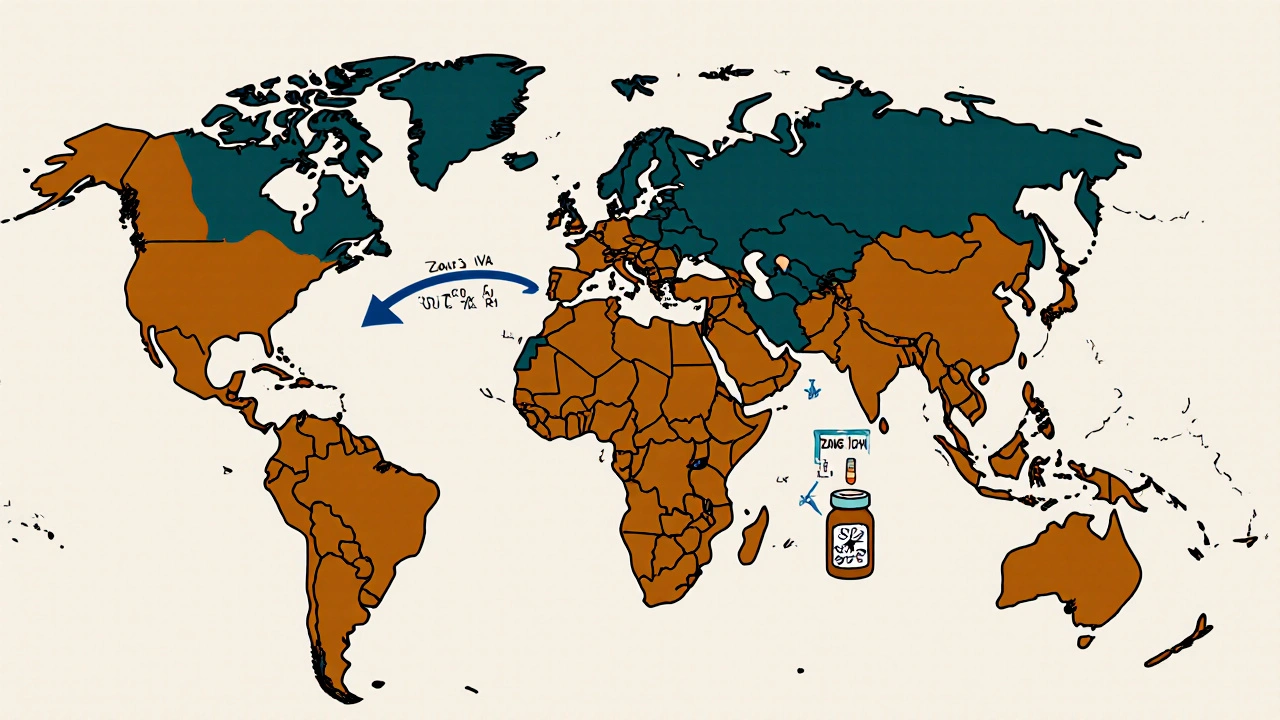
Drugs aren’t just sold in the UK or the U.S. They’re shipped to tropical countries, deserts, and high-humidity zones. That’s why ICH divides the world into five climatic zones:
- Zone I (Temperate): 21°C / 45% RH
- Zone II (Subtropical): 25°C / 60% RH
- Zone III (Hot-Dry): 30°C / 35% RH
- Zone IVa (Hot-Humid): 30°C / 65% RH
- Zone IVb (Hotter & Humid): 30°C / 75% RH
If you’re selling a drug in India, Brazil, or Nigeria (Zone IVa), you must prove it’s stable at 30°C and 65% RH for 12 months. That means some companies run parallel stability studies for different markets. One product, multiple protocols. That adds 4-6 months to development time and increases costs significantly.
What Happens When Conditions Go Wrong
Stability chambers aren’t just big fridges. They’re precision instruments. They must hold temperature within ±0.5°C and humidity within ±2% RH. Any drift outside that range can invalidate months of testing.
Real-world data shows this isn’t rare. In a 2023 survey of 142 pharmaceutical professionals, 78% reported at least one temperature excursion-meaning the chamber went above or below the allowed range. One 2°C spike during a 12-month study can mean restarting the whole test. That’s a $50,000 loss in time and materials.
Even small changes matter. A 4.8% drop in active ingredient concentration might sound minor, but if it falls below 95%, regulators can reject the batch-even if the change isn’t statistically significant. One Pfizer employee shared a case where a single failed test delayed a generic drug launch by eight months because the regulator didn’t accept their statistical argument.
Why the Rules Are Falling Behind
The ICH Q1A(R2) guidelines are nearly 20 years old. They were designed for pills and capsules. They don’t handle modern drugs well.
Take mRNA vaccines or monoclonal antibodies. These are fragile. A single freeze-thaw cycle can destroy them. But the standard 40°C/75% RH test doesn’t predict that. In 2021 and 2022, the FDA issued warning letters to Amgen and Roche for stability failures in biologics-because the old tests didn’t catch the problem.
Another issue: humidity. Most tests assume constant humidity. But in real life, humidity swings. A drug might sit in a dry warehouse, then get shipped in a humid truck, then stored in a steamy pharmacy. A 2022 AAPS paper found that 62% of solid dosage failures were due to humidity cycling, not constant conditions.
Experts like Dr. Lisa McLeod argue the system is outdated. “The ICH guidelines were never meant for lipid nanoparticles or cell therapies,” she wrote in her 2021 book. “We’re using a 1990s tool to test 2020s medicine.”
How Companies Are Adapting
Some companies are pushing beyond the rules. Merck used intermediate testing at 30°C/65% RH to catch a polymorphic shift in Keytruda®-a change that could’ve affected how the drug was absorbed. That discovery prevented a major recall.
Others are using predictive modeling. Instead of waiting 12 months, they run tests at 50°C, 60°C, even 80°C, and use software to predict degradation. Top pharmaceutical companies are using these Accelerated Predictive Stability (APS) studies. They can cut time-to-market by 9-12 months.
But regulators aren’t ready. In 2022-2023, the EMA rejected eight model-based stability submissions because they didn’t meet the old physical testing standard. The gap between science and regulation is widening.
What’s Next
The ICH is working on a new version-Q1F-expected by late 2024. It’s supposed to cover complex products like antibody-drug conjugates and cell therapies. The FDA is also testing real-time stability using process analytical technology (PAT). If it works, it could reduce testing time by 30-50% for drugs made with continuous manufacturing.
But for now, the rules stay the same. If you’re developing a drug, you still need to run the 40°C/75% RH test for six months. You still need 12 months of data at 25°C or 30°C. You still need to document every temperature reading, every humidity spike, every failed batch.
It’s tedious. It’s expensive. It takes years. But it’s the only thing standing between a patient and a broken pill. And in pharmaceuticals, there’s no room for shortcuts.
What are the standard temperature and humidity conditions for long-term stability testing?
The ICH Q1A(R2) guidelines allow two options for long-term stability testing of most solid oral dosage forms: either 25°C ± 2°C with 60% RH ± 5% RH, or 30°C ± 2°C with 65% RH ± 5% RH. The choice depends on the target market’s climate zone. Data must cover at least 12 months before regulatory submission, though the EMA permits 6 months under certain conditions.
Why is accelerated testing done at 40°C and 75% RH?
The 40°C/75% RH condition was chosen to simulate extreme but plausible environmental stress-like a drug left in a hot truck or warehouse during summer. It’s not meant to reflect normal storage. Instead, it accelerates degradation to predict long-term behavior. Studies show this condition roughly correlates to 24 months at 25°C/60% RH for most small-molecule drugs, though it’s less reliable for hygroscopic or biologic products.
Do refrigerated drugs follow the same stability rules?
No. Refrigerated products, like injectables and some biologics, are tested at 5°C ± 3°C for long-term stability. Their accelerated test is conducted at 25°C ± 2°C with 60% RH ± 5% RH-not the 40°C used for room-temperature drugs. This is because freezing and thawing cycles, not heat, are the main risk for these products. WHO Annex 10 and FDA guidance both support this distinction.
What happens if a stability chamber has a temperature excursion?
Any temperature or humidity deviation outside ±0.5°C or ±2% RH can invalidate the entire study. If the excursion lasts more than a few hours, regulators may require restarting the test from day one. In practice, 78% of pharmaceutical professionals report at least one such failure during a 12-month study. These incidents delay approvals, increase costs, and can trigger regulatory scrutiny.
Are ICH stability guidelines the same worldwide?
Yes, in principle. The ICH Q1A(R2) guidelines are adopted by the FDA, EMA, Health Canada, and other major agencies. However, differences exist in submission requirements-for example, the FDA requires 12 months of long-term data at submission, while the EMA allows 6 months under Option B. Zone-specific testing for tropical markets also adds complexity, requiring separate protocols for global products.
Why are stability tests so slow? Can’t we speed them up?
Traditional stability testing takes years because it’s designed to mimic real-world aging. But new approaches are emerging. Predictive modeling using higher temperatures (up to 80°C) and advanced analytics can estimate degradation in weeks. The FDA is piloting real-time stability testing using process analytical technology (PAT), which could reduce testing time by 30-50% for continuous manufacturing products. However, regulators still require physical data for approval, so full replacement isn’t yet possible.
Final Thoughts
Stability testing isn’t glamorous. It doesn’t make headlines. But it’s the quiet guardrail that keeps medicines safe. The rules are old, the tests are slow, and the equipment is expensive. Yet, every temperature reading, every humidity log, every failed batch is part of a system that’s kept patients safe for decades.
As drugs get more complex-mRNA vaccines, gene therapies, personalized medicines-the old system will keep being tested. The next decade will bring change. But until then, the numbers don’t change: 25°C, 40°C, 12 months, 6 months. They’re not arbitrary. They’re the result of decades of science, failure, and hard lessons learned. Get them right. Because someone’s health depends on it.

11 Comments
Scott MacfadyenNovember 19, 2025 AT 14:58
Man, I’ve seen stability chambers fail so hard it’s ridiculous. One time, a 2°C spike during a 12-month test meant we had to restart everything. $50K down the drain. No one talks about how much of this is just expensive guesswork dressed up as science.
Denise CauchonNovember 20, 2025 AT 06:06
THEY STILL USE 1990s RULES FOR 2024 MEDS?? 😭 I mean… come ON. mRNA vaccines need a whole new playbook, not some dude in a lab in 2003 thinking ‘40°C = bad’ and calling it a day. This isn’t testing-it’s a religious ritual with thermometers.
Andrea JohnstonNovember 20, 2025 AT 06:34
Let’s be real: this entire system is a monument to bureaucratic inertia. We’ve got AI models that can predict degradation with 95% accuracy in weeks, but regulators still demand 12 months of physical data because ‘that’s how it’s always been.’ It’s not science-it’s compliance theater with a side of expired aspirin.
Alex CzartoryskiNovember 21, 2025 AT 15:36
And yet somehow, the same people who demand 12 months of data won’t let you use a $200 sensor to log temp in real time. It’s like they want us to fail. I’ve seen labs use analog thermometers because ‘the software doesn’t meet FDA specs.’ We’re running a space program with a slide rule.
Brandon LowiNovember 22, 2025 AT 07:31
Let me tell you something-this isn’t about science. It’s about control. The FDA doesn’t want innovation. They want compliance. They want paperwork. They want you to beg for permission to make a better drug. And guess what? The only thing more dangerous than a bad pill? A regulator who thinks he’s God.
Chloe SevignyNovember 23, 2025 AT 16:39
The ICH Q1A(R2) framework was never designed for lipid nanoparticles, monoclonal antibodies, or gene therapies. It was designed for aspirin in a glass bottle. To pretend it’s still adequate is not just outdated-it’s epistemologically negligent. The regulatory apparatus is operating under a paradigm that has been rendered obsolete by the very molecules it was meant to govern.
Gizela CardosoNovember 25, 2025 AT 10:57
I work in a lab that tests for tropical markets. We run parallel studies for India and Canada. It’s exhausting, but it works. I just wish the system didn’t make us jump through so many hoops just to keep people alive. Maybe one day we’ll stop treating patients like afterthoughts.
Alex BoozanNovember 27, 2025 AT 09:01
And don’t get me started on humidity cycling. The guidelines assume constant RH? That’s not reality-that’s a fantasy written by someone who’s never left a climate-controlled lab. A drug sits in a dry warehouse, gets shipped in a humid truck, then ends up in a steamy pharmacy in Manila. The degradation isn’t linear-it’s chaotic. And the regulators? They still demand a single number.
Richard CouronNovember 28, 2025 AT 07:10
They’re lying to us. EVERYTHING is rigged. The 40°C test? It’s not for safety-it’s for profit. Big Pharma wants to delay generics. That’s why they make you wait 12 months. That’s why they push ‘validation.’ They don’t care if the drug works-they care if you pay them to prove it. Wake up. The expiration date is a scam.
Victoria MalloyNovember 29, 2025 AT 05:37
I just want to say thank you to everyone who logs these temps, runs these tests, and doesn’t cut corners. It’s boring, it’s slow, and no one sees it-but it’s what keeps my grandma’s blood pressure meds from turning into poison. You’re the real heroes.
Joshua CasellaNovember 29, 2025 AT 15:04
Chloe nailed it. The system is broken. But here’s the thing-we don’t need to burn it down. We need to upgrade it. The FDA’s PAT pilot? That’s the future. Real-time data. AI modeling. Less waiting. More accuracy. Let’s push for this. Not against regulators. With them. Because if we get this right, it doesn’t just save time-it saves lives.