Every time you pick up a prescription at the pharmacy and see a brand name you don’t recognize-like apixaban instead of Eliquis-you’re seeing the result of something called an ANDA. It’s not a drug. It’s not a company. It’s a paperwork shortcut that saves you and the healthcare system billions every year. The Abbreviated New Drug Application is how generic drugs get approved in the U.S., and it’s one of the most effective public health tools you’ve never heard of.
What exactly is an ANDA?
An ANDA, or Abbreviated New Drug Application, is the official form a company submits to the U.S. Food and Drug Administration (FDA) to get permission to sell a generic version of a brand-name drug. The word "abbreviated" is key here. Unlike the original drug maker, who had to run full clinical trials to prove their medicine was safe and effective, a generic company doesn’t have to start from scratch. They can use the FDA’s existing data on the brand-name drug-called the Reference Listed Drug (RLD)-to prove their version works the same way.
This system wasn’t always in place. Before 1984, generic drugs faced huge legal and scientific barriers. The Hatch-Waxman Act changed that. Signed by President Ronald Reagan on September 24, 1984, it created two paths: one for brand-name drugs (NDA) and one for generics (ANDA). The goal? Make medicines cheaper without sacrificing safety. And it worked. By 2023, over 11,000 generic drugs had been approved through ANDAs, making up about 90% of all prescriptions filled in the U.S.
How does an ANDA prove a generic drug works?
Generic drugs don’t need to repeat animal or human trials. Instead, they must prove two things: pharmaceutical equivalence and bioequivalence.
Pharmaceutical equivalence means the generic has the same active ingredient, strength, dosage form (like tablet or injection), and route of administration (oral, topical, etc.) as the brand-name drug. That’s straightforward. But bioequivalence is where the real science kicks in.
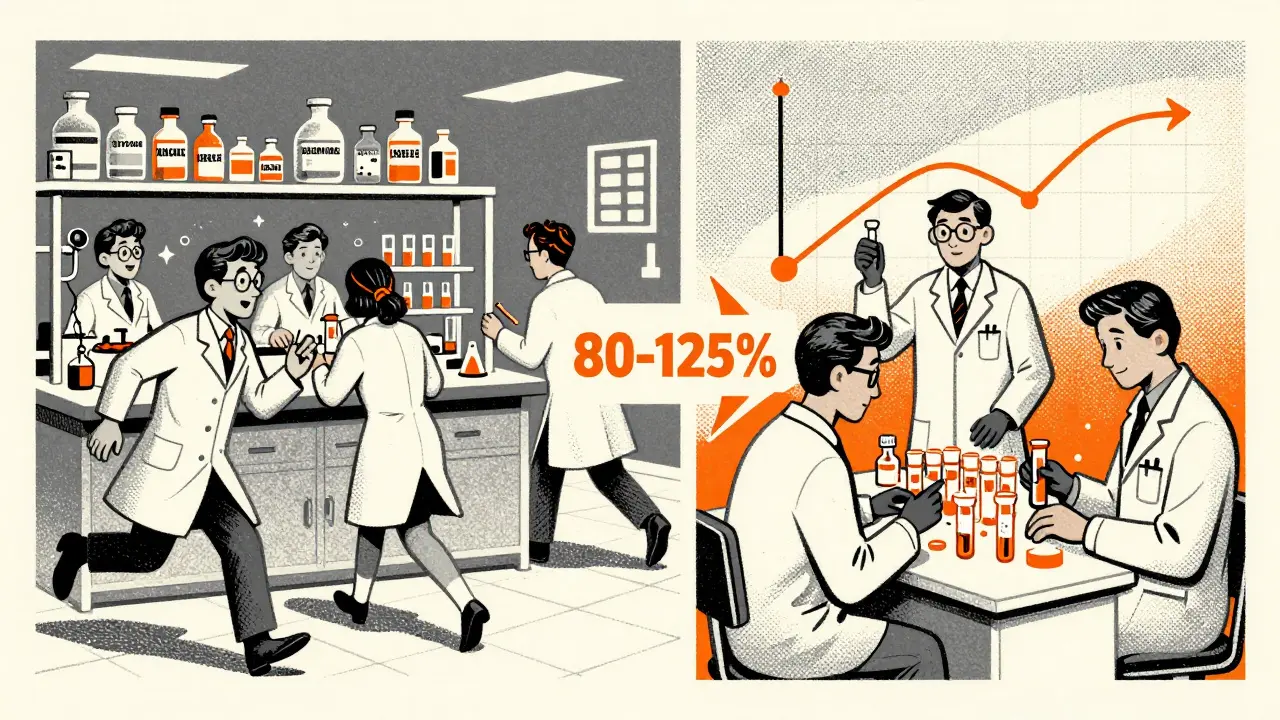
To prove bioequivalence, a generic manufacturer runs a study with 24 to 36 healthy volunteers. These people take both the brand-name drug and the generic version, and researchers measure how much of the drug enters their bloodstream and how fast. The key numbers are AUC (area under the curve) and Cmax (maximum concentration). For the generic to be approved, the 90% confidence interval for these measurements must fall between 80% and 125% of the brand-name drug’s results.
That range might sound wide, but it’s scientifically tight enough to ensure no meaningful difference in how the drug works in the body. The FDA has found that 97% of generic drugs approved this way are therapeutically equivalent to their brand-name counterparts. In other words, if your doctor prescribes Lipitor and you get the generic atorvastatin, your cholesterol levels won’t know the difference.
What’s NOT allowed in an ANDA?
Not every drug can go generic. The ANDA pathway only works for drugs that are simple enough to copy exactly. That means small-molecule pills, capsules, and injections with well-understood absorption patterns. Complex drugs like inhalers, topical creams, or injectables with nanoparticles often can’t be approved through ANDA because their effects depend on how they’re delivered, not just what’s inside.
For those, the FDA has special programs, like the Complex Generic Drug Product Development initiative launched in 2022. But even then, approval takes longer and costs more. That’s why most ANDAs are for common pills-blood pressure meds, statins, antibiotics, and diabetes drugs.
Another thing you won’t find in an ANDA: new claims. A generic drug can’t say it’s "better," "faster," or "more effective" than the brand. Its labeling must match the brand’s exactly-except for the company name and logo. You can’t change the warnings, dosage instructions, or side effect list. The FDA requires this to prevent misleading patients.
How is an ANDA different from an NDA?
Here’s the big contrast:
- NDA (New Drug Application): Used by brand-name companies. Requires full preclinical (animal) and clinical (human) trials. Takes 10-15 years. Costs around $2.6 billion.
- ANDA (Abbreviated New Drug Application): Used by generic companies. Uses existing data. Takes 3-4 years. Costs $1-5 million.
The FDA’s review time reflects this too. A standard NDA takes 12 months to review. A priority NDA (for urgent needs) takes 10 months. An ANDA? Also 10 months-thanks to the Generic Drug User Fee Amendments (GDUFA), which gave the FDA more funding to speed up reviews.
That’s why generics hit the market so fast after a brand patent expires. In 2022 alone, the FDA approved 724 new generic drugs. Those approvals were projected to save the U.S. healthcare system $23.7 billion in one year.
Who benefits from ANDAs?
Everyone does.
Patients pay less. Generic drugs cost 80-85% less than brand-name versions within a year of launch. The average annual savings per prescription is $150-$300. For someone on multiple medications, that adds up to thousands a year.
Insurance companies and government programs like Medicare and Medicaid save billions. In 2023, generic drugs saved the U.S. healthcare system $313 billion-according to the Association for Accessible Medicines. That’s more than the entire annual budget of the Department of Education.
Even the brand-name companies benefit indirectly. The ANDA system gives them a predictable window of exclusivity-usually 10-12 years-before generics enter. That’s their time to recoup R&D costs. After that, competition keeps prices down, which keeps public pressure off them to raise prices.
And let’s not forget the manufacturers. Companies like Teva, Mylan (now Viatris), and Sandoz built entire businesses around ANDAs. Together, they control nearly half the U.S. generic market. Smaller companies use ANDAs to enter the market without needing a billion-dollar budget.
What goes wrong with ANDAs?
It’s not perfect. About 32% of ANDAs get rejected on their first try, mostly because of manufacturing issues. The FDA looks closely at how the drug is made-cleanliness of facilities, consistency of batches, stability over time. If a company can’t prove their generic won’t break down in a hot warehouse or lose potency after six months, the application gets a "complete response letter."
Another common problem: bioequivalence data that doesn’t meet the 80-125% range. This happens more often with complex dosage forms, like extended-release pills or oral suspensions. In 2022, 27% of rejections were tied to bioequivalence concerns.
And then there’s the patent game. The Hatch-Waxman Act lets generic companies challenge patents on brand-name drugs. If they do, they get 180 days of exclusive rights to sell the first generic version. That’s a huge incentive. But it also leads to legal battles. A single patent dispute can delay a generic launch for years.
What’s changing in the ANDA world?
The FDA is pushing for more first-time approvals. Under GDUFA IV, set to run through 2027, the goal is to approve 90% of original ANDAs on the first try-up from 65% today. That means clearer guidance, faster feedback, and more support for smaller companies.
There’s also a push to approve more complex generics. Inhalers, topical antifungals, and injectable gels are the next frontier. The FDA released new guidance in 2022 to help manufacturers navigate these harder-to-copy products.
But there’s a warning: supply chain risk. Over 80% of generic drug ingredients come from India and China. A single factory shutdown-whether from weather, politics, or quality issues-can cause nationwide shortages. Experts like Dr. Rachel Sherman have called this a systemic vulnerability.
Still, the numbers don’t lie. The Congressional Budget Office projects that between 2024 and 2033, generic drugs will save the U.S. healthcare system $1.7 trillion. That’s not just a policy win. It’s a lifeline for millions of people who can’t afford brand-name drugs.
Why does this matter to you?
If you’ve ever paid for a prescription, you’ve felt the impact of ANDAs. That $4 generic statin? That’s the Hatch-Waxman Act in action. That $100 insulin? Without generics, it could be $300. The ANDA system isn’t flashy. It doesn’t make headlines. But it’s the quiet engine behind affordable medicine in America.
It’s not about cutting corners. It’s about using science wisely. If a drug works in one form, why reinvent it? The FDA doesn’t approve generics lightly. Every one must meet the same high standards as the brand. The only difference? The price tag.
Is an ANDA the same as a generic drug?
No. An ANDA is the application submitted to the FDA to get approval for a generic drug. The generic drug is the actual medicine you take. Think of the ANDA as the paperwork, and the generic drug as the product that results from it.
Can any drug become a generic through ANDA?
No. Only drugs that are chemically simple and have well-understood absorption patterns qualify. Complex drugs like inhalers, biologics, or certain injectables often require different approval pathways. Biologics, for example, go through a separate process called biosimilar approval under the BPCIA.
How long does it take to get an ANDA approved?
The FDA aims to review a standard ANDA within 10 months under the GDUFA program. But the whole process-from developing the drug to submitting the application-usually takes 3 to 4 years. Many applications are rejected on the first try, so companies often resubmit after fixing issues, which adds more time.
Do generic drugs work as well as brand-name drugs?
Yes. The FDA requires generics to be therapeutically equivalent to the brand-name drug. Studies show that 97% of generic drugs have the same clinical effect. Millions of people take generics every day without any difference in how they feel or how their condition is managed.
Why are generic drugs so much cheaper?
Because generic manufacturers don’t have to pay for expensive clinical trials or marketing campaigns. They rely on the brand’s existing safety and efficacy data. Their costs are mostly in manufacturing and regulatory submission. That’s why a generic version of a drug can cost 80-85% less than the brand.
Are ANDAs only used in the United States?
The term "ANDA" is specific to the U.S. FDA. Other countries have similar systems but different names. The European Medicines Agency uses "abridged applications," and Health Canada has "generic drug submissions." The core idea-using existing data to approve copies-is used worldwide, but the rules and names vary.
What’s next for generic drugs?
The future of ANDAs isn’t about more pills. It’s about better ones. As more complex drugs come off patent-like biosimilars for cancer treatments and advanced inhalers-the FDA is adapting. The goal is to keep the ANDA pathway strong for simple generics while building new tools for harder-to-copy medicines.
One thing won’t change: the need for affordable drugs. With healthcare costs rising and chronic diseases growing, the ANDA system remains the most reliable way to keep medicines within reach. It’s not perfect. But it’s proven. And for millions of people, it’s the reason they can afford to stay healthy.

13 Comments
Nancy KouDecember 20, 2025 AT 13:57
Generic drugs saved my dad’s life. He’s on five meds and the brand names would’ve cost $1,200 a month. The generics? $45. No difference in how he feels. The system works.
Meenakshi JaiswalDecember 20, 2025 AT 18:25
For anyone worried about generics not working: the FDA’s bioequivalence standards are brutal. 80-125% isn’t a loophole-it’s a tight margin designed to catch even tiny differences. If your generic feels off, it’s likely the filler, not the active ingredient.
Dev SawnerDecember 22, 2025 AT 15:52
The assertion that ANDAs are a public health triumph is statistically misleading. While cost savings are undeniable, the regulatory capture by Indian and Chinese API manufacturers has created systemic vulnerabilities. The 2018 heparin contamination incident was not an anomaly-it was an inevitability of outsourcing quality control to jurisdictions with lax oversight. The FDA’s inspection capacity remains woefully inadequate to monitor 80% of global production.
Andrew KellyDecember 24, 2025 AT 04:52
Let’s be real-this whole system is a corporate shell game. The brand-name companies pay the FDA millions through user fees, then lobby to extend patents with frivolous tweaks. Meanwhile, generics get slapped with 32% rejection rates for minor manufacturing flaws. Who’s really benefiting? Not you. Not me. The lawyers and the FDA contractors.
Monte PareekDecember 25, 2025 AT 08:32
Look I’m from the Midwest and I’ve been taking generics since 2008. I’ve taken atorvastatin levothyroxine metformin you name it. No side effects no weird reactions. My doctor says they’re identical. My wallet says thank you. Stop overthinking it. The science is solid.
Also if you think your brand name drug is better you’re probably paying for the ads not the medicine
Hussien SLeimanDecember 26, 2025 AT 06:30
Of course the FDA approves generics-they’re not trying to kill people, they’re trying to keep the system from collapsing under its own weight. But let’s not pretend this is some noble public service. The Hatch-Waxman Act was written by pharmaceutical lobbyists who wanted to lock in 12 years of monopoly pricing before letting the cheap copies in. It’s not about access-it’s about controlled competition. And now we’ve got a two-tiered system where the rich get the branded stuff with fancy packaging and the rest of us get the same pills in a plain bottle with a 20% higher failure rate in stability testing. Don’t believe me? Look up the FDA’s 2021 inspection reports on Indian facilities. Half of them had critical violations. Yet they still get approved. Why? Because the backlog is so massive they have to greenlight half the applications just to stay on schedule. It’s not a triumph of efficiency-it’s triage disguised as policy.
Connie ZehnerDecember 27, 2025 AT 03:41
I used to take the generic for my anxiety med and I swear I felt worse. Like my brain was foggy and I couldn’t focus. I switched back to brand and boom-back to normal. So yeah maybe 97% work but what about the 3% who actually feel the difference? Nobody talks about that. And why do the side effects always seem worse on generics? Is it the dye? The filler? I don’t know but I’m not risking it again.
holly SinclairDecember 27, 2025 AT 15:08
If we’re going to celebrate the ANDA system as a triumph of rationality, we must also ask: what does it mean to say a drug is "the same"? Is sameness purely chemical? Or does the placebo effect, the branding, the ritual of taking a pill with a familiar logo, play a role in therapeutic outcome? The FDA measures blood concentrations, but not belief. And belief matters. A patient who trusts their brand-name drug may experience better outcomes-not because the chemistry differs, but because their psyche does. The ANDA system reduces medicine to a mathematical equation. But humans aren’t equations. We’re stories wrapped in biology. And sometimes, the story we tell ourselves about our medicine is as important as the molecule itself.
Moses OdumbeDecember 27, 2025 AT 20:05
GENERIC DRUGS ARE LITERALLY THE SAME 💯
the FDA doesn't mess around. if your generic doesn't hit 80-125% bioequivalence it gets rejected. period.
also the fact that you think brand-name is "better" is just marketing brainwashing. you're paying for the color of the pill and the jingle not the science. 🧪💸
Elaine DouglassDecember 28, 2025 AT 23:15
i just want to say thank you to everyone who makes these drugs possible. my mom takes 7 generics every day and without them she wouldn't be here. it's not glamorous but it's everything
Tim GoodfellowDecember 29, 2025 AT 14:22
Anda? More like "Ain’t No Drama Act"-because it’s the quiet hero that keeps the system from imploding. No fanfare, no IPO, no influencer endorsements. Just pills. Cheap, clean, and critical. If the FDA were a band, ANDA would be the bassist: nobody notices them until the song falls apart.
Mahammad MuradovDecember 30, 2025 AT 14:08
It is a well-documented fact that the bioequivalence range of 80-125% permits pharmacokinetic variance that may result in subtherapeutic or toxic exposure in sensitive populations, particularly the elderly and those with hepatic impairment. The FDA’s reliance on healthy volunteer studies is methodologically insufficient to ensure population-wide therapeutic equivalence. Furthermore, the absence of post-marketing pharmacovigilance requirements for generics creates a dangerous regulatory blind spot. This is not innovation-it is institutionalized risk.
Isabel RábagoDecember 30, 2025 AT 17:36
It’s not just about cost. It’s about integrity. When you let a drug manufacturer skip clinical trials and just copy a formula, you’re not saving money-you’re eroding the foundation of medical trust. People don’t realize that every time they take a generic, they’re betting their health on a factory in Hyderabad or Shanghai. And if that factory cuts corners? Millions of people pay the price. This isn’t efficiency. It’s gambling with human lives.